- Home
- Green Building Tourism Factory
- Introduction
- Manufacturing Process Display Gallery
國際規格製程
To ensure top-quality products, Biotanico has established a rigorous manufacturing process control system. Our production chain follows internationally recognized standards, including GMP、HACCP、ISO 22000、ISO 9001 ith dozens of critical quality control checkpoints throughout the process. Through the close collaboration between frontline operators and quality control specialists, we are able to produce high-standard health products that meet the highest global standards
- GMP
- HACCP
- ISO 22000
- ISO 9001
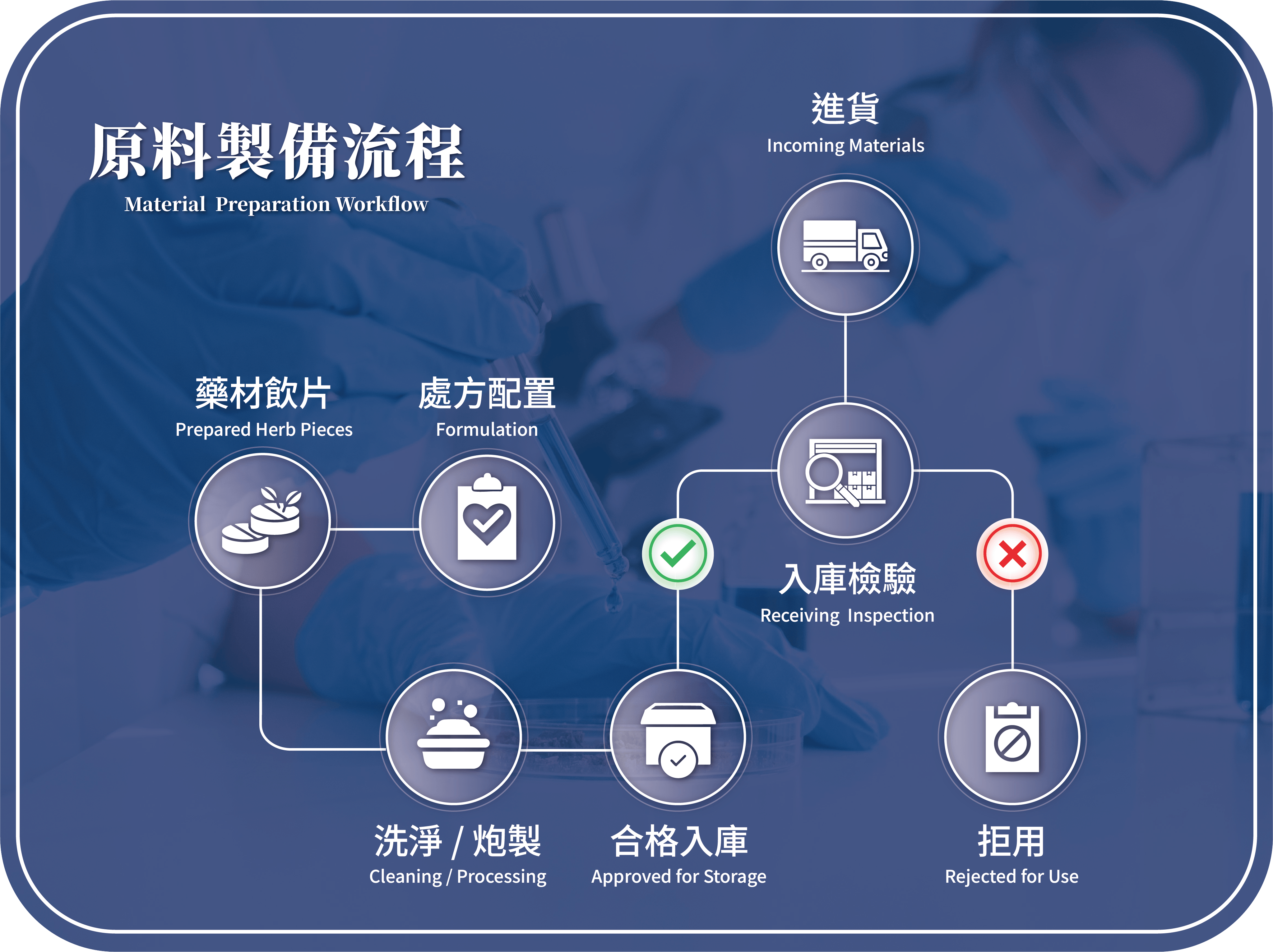
- Raw Material Stage:Suppliers Evaluation, incoming inspection, traceability management, and clearly defined of acceptance and rejection standard
- Production Stage: Cleaning/ preparation, formula compounding, and monitoring and documentation at critical control points
- Releasing Stage:Inspection of semi-finished and finished products; complete sampling and traceability records maintained
Batch-by-Batch Quality Control

At Biotanico, with a “Quality First” manufacturing philosophy, we rigorously implement every step of dosage form production and packaging to ensure that each product meets international standards from manufacturing to warehouse storage. The entire process begins with dosage form manufacturing and proceeds through strict in-process quality control (IPQC) for semi-finished products. Only batches that meet quality requirements move on to the secondary packaging stage. Multiple quality control checkpoints are established at every stage, and any non-conforming products are immediately rejected. After packaging, products undergo final quality control (FQC), with comprehensive tests conducted according to GMP and ISO 22000 standards to ensure content stability, safety, and compliance with specifications. Only then are products cleared for storage and eventual shipment.
- • Every process is documented.
- • Every stage is inspected.。
- • Every product is an extension of trust
Advanced Manufacturing Technology
Building on pharmaceutical-grade expertise, Biotanico has implemented fluidized bed spray drying and granulation using imported Japanese non-continuous granulation equipment. This closed-system process completes spray drying and granule formation, providing superior absorption compared to traditional methods. Additionally, the facility is equipped with automatic capsule filling machines, fully automated tablet presses, and fully automated PTP blister packaging machines, ensuring that health supplements are produced with the highest standards of safety, excellence, and quality.


Herbal Extraction Expertise
Natural herbs contain a variety of polar and non-polar active compounds that require different solvents or extraction methods to isolate effectively. With decades of experience in herbal extraction, Biotanico has developed herbal essential oil extraction technology, which has been awarded Taiwan Patent I381876. This method ensures that the raw materials retain a full spectrum of active compounds throughout the extraction process.
- Water-Soluble Active Compounds:Goji polysaccharides(Polysaccharides),Hawthorn organic acids (Organic acids) ,Honeysuckle flavonoids (Flavonoids),Ginseng saponins (Saponins)
- Fat-Soluble Active Compounds:Peppermint essential oils (Essential oils),Goji Vitamin A (Carotenoids)
Herbal Health Solutions for the Whole Family
In response to diverse market needs, Biotanico has established production lines for up to 24 dosage forms, fully catering to the health and wellness needs of the entire family.
- •Traditional Dosage Forms: Tablets, capsules, powders, oral liquids, pills, medicated plasters, topical powders, fragments, ointments, granules
- •Concentrated Dosage Forms: Concentrated tablets, concentrated capsules, concentrated powders, concentrated granules.
- •Raw Material Dosage Forms:Extracts, liquid extracts